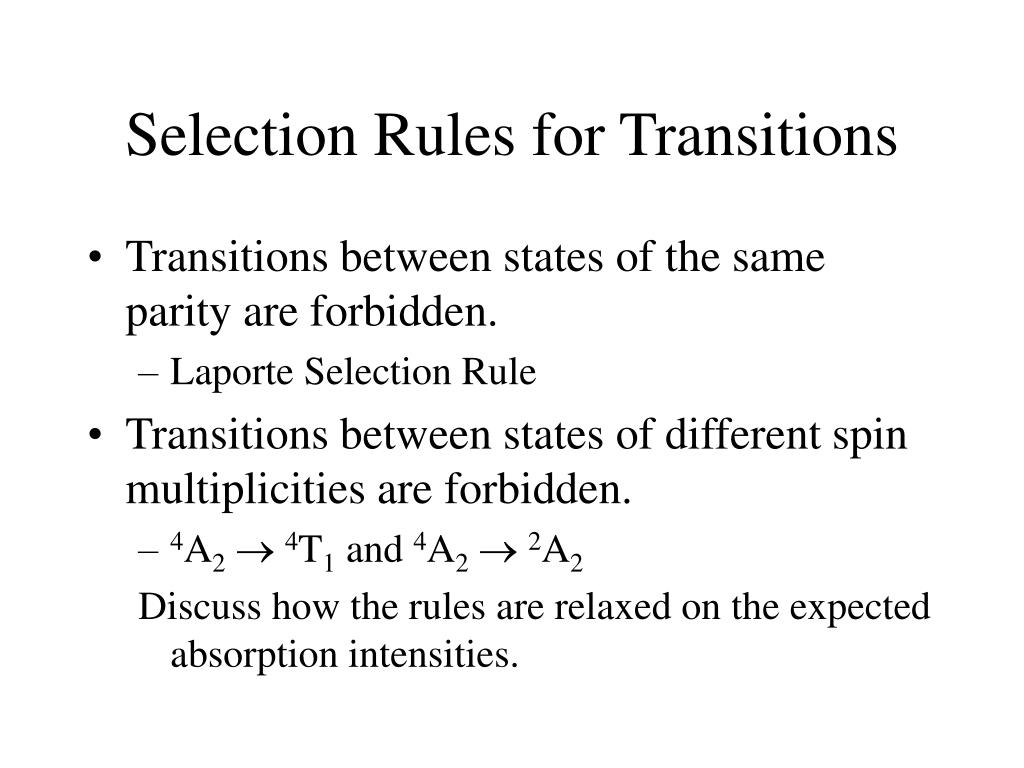
D-D Transition Selection Rules . the selection rules governing transitions between electronic energy levels of transition metal complexes are: It is a selection rule that rigorously. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. the selection rules governing transitions between electronic energy levels (including microstates and terms) are: ligand field or dd transitions. A change in parity occurs i.e. \(∆ l = + 1\) laporte allowed transitions: Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. • the stability of the bonding comes from the ligand orbitals being. the laporte rule is a rule that explains the intensities of absorption spectra for chemical species.
from www.slideserve.com
Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. A change in parity occurs i.e. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. It is a selection rule that rigorously. the selection rules governing transitions between electronic energy levels (including microstates and terms) are: the selection rules governing transitions between electronic energy levels of transition metal complexes are: the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. • the stability of the bonding comes from the ligand orbitals being. ligand field or dd transitions. \(∆ l = + 1\) laporte allowed transitions:
PPT Coordination Chemistry III Electronic Spectra PowerPoint Presentation ID1223870
D-D Transition Selection Rules the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. the selection rules governing transitions between electronic energy levels (including microstates and terms) are: ligand field or dd transitions. • the stability of the bonding comes from the ligand orbitals being. \(∆ l = + 1\) laporte allowed transitions: For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. A change in parity occurs i.e. It is a selection rule that rigorously. the selection rules governing transitions between electronic energy levels of transition metal complexes are: Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear.
From www.slideserve.com
PPT Motivation PowerPoint Presentation, free download ID4418328 D-D Transition Selection Rules A change in parity occurs i.e. \(∆ l = + 1\) laporte allowed transitions: ligand field or dd transitions. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. It is a selection rule that rigorously. Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a. D-D Transition Selection Rules.
From www.numerade.com
SOLVED The electronic configuration and geometry of a complex determine the intensities of its D-D Transition Selection Rules \(∆ l = + 1\) laporte allowed transitions: the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. the selection rules governing transitions between electronic energy levels (including microstates and terms) are: For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,.. D-D Transition Selection Rules.
From www.slideserve.com
PPT The Electronic Spectra of Coordination Compounds PowerPoint Presentation ID463738 D-D Transition Selection Rules Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. the selection rules governing transitions between electronic energy levels of transition metal complexes are: It is a selection rule that rigorously. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. . D-D Transition Selection Rules.
From www.youtube.com
20 6 Selection Rules Why dd Transitions Are Observed YouTube D-D Transition Selection Rules Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. ligand field or dd transitions. It is a selection rule that rigorously. the selection rules governing transitions between electronic energy levels. D-D Transition Selection Rules.
From www.youtube.com
dd संक्रमण,dd Transition,चक्रण चयन नियम,Spin selection Rule, unit8 bsc3rdsemesterchemistry D-D Transition Selection Rules • the stability of the bonding comes from the ligand orbitals being. the selection rules governing transitions between electronic energy levels of transition metal complexes are: ligand field or dd transitions. It is a selection rule that rigorously. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,.. D-D Transition Selection Rules.
From studylib.net
Selection Rules for electronic transitions D-D Transition Selection Rules \(∆ l = + 1\) laporte allowed transitions: ligand field or dd transitions. Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. the selection rules governing transitions between electronic energy levels (including microstates. D-D Transition Selection Rules.
From chem.libretexts.org
Selection rules and transition moment integral Chemistry LibreTexts D-D Transition Selection Rules For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. the selection rules governing transitions between electronic energy levels (including microstates and terms) are: • the stability of the bonding comes from the ligand orbitals being. A change in parity occurs i.e. ligand field or dd transitions. It. D-D Transition Selection Rules.
From www.chegg.com
Solved (i) State all of the selection rules for an electric D-D Transition Selection Rules \(∆ l = + 1\) laporte allowed transitions: Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. It is a selection rule that rigorously. ligand field or dd transitions. the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. • the stability of the. D-D Transition Selection Rules.
From www.youtube.com
What is Laporte Orbital Selection Rule dd Transitions & Laporte & Spin Selection Rules YouTube D-D Transition Selection Rules the selection rules governing transitions between electronic energy levels (including microstates and terms) are: It is a selection rule that rigorously. A change in parity occurs i.e. Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. the selection rules governing transitions between electronic energy levels of transition metal complexes are:. D-D Transition Selection Rules.
From www.slideserve.com
PPT Properties of Transition Metals PowerPoint Presentation ID759793 D-D Transition Selection Rules • the stability of the bonding comes from the ligand orbitals being. the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. It is a selection rule that rigorously. the selection rules governing transitions between electronic energy levels (including microstates and terms) are: the selection rules governing transitions between electronic energy. D-D Transition Selection Rules.
From slidetodoc.com
The roles of orbital in the optical and D-D Transition Selection Rules the selection rules governing transitions between electronic energy levels (including microstates and terms) are: A change in parity occurs i.e. • the stability of the bonding comes from the ligand orbitals being. the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. ligand field or dd transitions. It is a selection. D-D Transition Selection Rules.
From www.youtube.com
B.Sc. III Year Selection rules for dd transitions dd संक्रमण के लिए चयन नियम YouTube D-D Transition Selection Rules It is a selection rule that rigorously. the selection rules governing transitions between electronic energy levels (including microstates and terms) are: the selection rules governing transitions between electronic energy levels of transition metal complexes are: \(∆ l = + 1\) laporte allowed transitions: For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ. D-D Transition Selection Rules.
From www.slideserve.com
PPT Lecture 36 Electronic spectroscopy PowerPoint Presentation ID2077297 D-D Transition Selection Rules ligand field or dd transitions. the selection rules governing transitions between electronic energy levels of transition metal complexes are: It is a selection rule that rigorously. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. • the stability of the bonding comes from the ligand orbitals being.. D-D Transition Selection Rules.
From www.slideserve.com
PPT Periodicity (AHL) PowerPoint Presentation, free download ID6265972 D-D Transition Selection Rules the selection rules governing transitions between electronic energy levels (including microstates and terms) are: \(∆ l = + 1\) laporte allowed transitions: A change in parity occurs i.e. the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. • the stability of the bonding comes from the ligand orbitals being. It is. D-D Transition Selection Rules.
From jwvulwxsqw.blogspot.com
Laporte Selection Rule / Selection Rules For Electronic Transitions Only allowed transitions D-D Transition Selection Rules the selection rules governing transitions between electronic energy levels of transition metal complexes are: the selection rules governing transitions between electronic energy levels (including microstates and terms) are: • the stability of the bonding comes from the ligand orbitals being. A change in parity occurs i.e. Electrons respond much faster than nuclear motion, therefore an excitation proceeds without. D-D Transition Selection Rules.
From www.researchgate.net
Transition diagrams showing dipole selection rules of the... Download Scientific Diagram D-D Transition Selection Rules ligand field or dd transitions. Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd (u,. \(∆ l = + 1\) laporte allowed transitions: the selection rules governing transitions between electronic energy. D-D Transition Selection Rules.
From chem.libretexts.org
Selection rules and transition moment integral Chemistry LibreTexts D-D Transition Selection Rules Electrons respond much faster than nuclear motion, therefore an excitation proceeds without a change to the nuclear. the laporte rule is a rule that explains the intensities of absorption spectra for chemical species. It is a selection rule that rigorously. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd. D-D Transition Selection Rules.
From www.youtube.com
🔥 Selection Rule For dd transition 💡 dd transition 🔥 d d transition 🔥 Laporte Selection Rule D-D Transition Selection Rules the selection rules governing transitions between electronic energy levels of transition metal complexes are: ligand field or dd transitions. \(∆ l = + 1\) laporte allowed transitions: • the stability of the bonding comes from the ligand orbitals being. For d orbitals, ψ(qs) and ψ’(es) will be of even (g, gerade) symmetry, but ˆ μ transforms as odd. D-D Transition Selection Rules.